Mar 4, 2024
A Novel Recipe for Regeneration & Recovery in Complete SCI
Sam Maddox
A new edition of CureCast dropped a couple of days ago, featuring a stimulating conversation with two young, creative scientists, Mark Anderson and Jordan Squair, the main authors of a bold and imaginative research study published last fall in the journal Science (Recovery of walking after paralysis by regenerating characterized neurons to their natural target region).
Anderson and Squair do a fine job explaining their step-by-step approach to spinal cord regeneration and recovery of function. This blog post offers a bit of background and context to a lively and engaging conversation with U2FP’s Matthew Rodreick and Jason Stoffer.
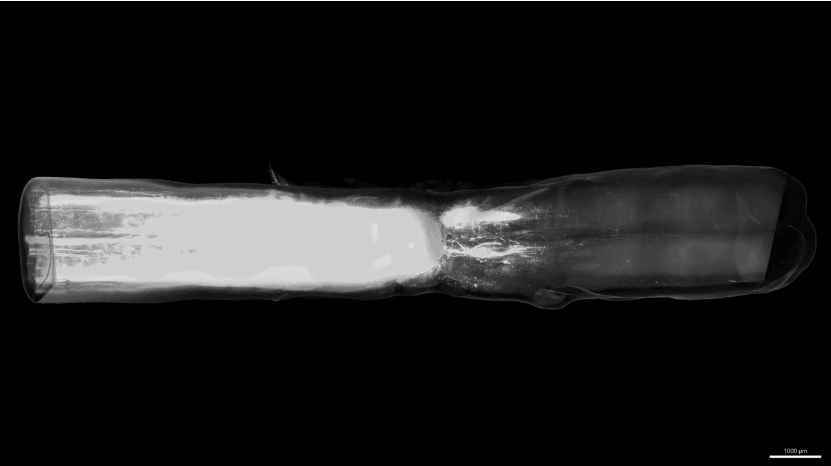
Working with their colleagues at the Swiss Federal Institute of Technology, UCLA and Harvard, Anderson and Squair took on one of the holy grail challenges of SCI research: repairing an anatomically complete injury. They report substantial recovery of walking function in 27 of 30 animals, using a complicated series of steps to mimic developmental neurobiology, that is, to turn on and guide growth of walking-specific spinal cord nerves to connect appropriately below the injury lesion site – just the way we formed the nervous system when we were infants
Get excited but keep it cool: This experiment uses an acute injury in a small animal – it’s not meant to be a clinically relevant study, tempting as it is to jump the presumptive shark.
Several caveats:
- Mice began the treatment protocol by receiving a virus-ferried genetic manipulation to specific cord neurons two weeks before being paralyzed, allowing time for molecular activation of nerve growth;
- The injuries involve a two-stage mid-thoracic hemi-section crush (half the cord is injured, then eight weeks later another crush is applied opposite the first one), a type of injury that would never occur except in a lab experiment;
- Regenerating axons didn’t have to travel very far in the lumbar cord to connect to motor nerves; clinical SCI will require long-distance growth.
Listen, we’re not holding this paper up to meet anyone’s translational expectations. Speculate at your own risk – one can’t not imagine the clinical possibilities. But keep in mind, this is a proof of concept study. There’s huge upside, but please, at this point there are many more questions than answers.
Anderson and Squair
Self-described as best friends, Anderson and Squair came to Switzerland about ten years ago as post-graduate students under Gregoire Courtine (who had a major role in the new study). Anderson, who is from Los Angeles, came from UCLA and the Michael Sofroniew lab, working with astrocytes and regenerative biology (Sofroniew also had a key role in the study).
Squair, a Canadian, trained in Vancouver with Andrei Krassioukov, mainly focused on electrical stimulation studies related to blood pressure and spinal cord injury. Squair won the 2023 grand prize from the BioInnovation Institute & Science Prize for Innovation, recognizing his work with orthostatic hypotension, “at the intersection of the life sciences and entrepreneurship.”
You will hear them mention a 2018 paper in the journal Nature. That paper, from Anderson and Sofroniew, developed the basic regeneration recipe: turn on the dormant genes in a specific type of spinal nerve (a spinal interneuron called Vsx2); induce a growth-supportive pathway/matrix with various growth factor molecules; and stimulate targeted growth using chemicals to attract axon growth.
From the paper:
“ . . . overcoming the failure of axon regrowth across anatomically complete SCI lesions after maturity required the combined sequential reinstatement of several developmentally essential mechanisms that facilitate axon growth. These findings identify a mechanism-based biological repair strategy for complete SCI lesions that could be suitable to use with rehabilitation models designed to augment the functional recovery of remodeling circuits.”
Fine work. The axons grew and crossed the lesion site, but they didn’t connect. There was no recovery.
Now, five years later, Anderson and Squair herein describe how they addressed the missing connection using another genetic process to produce a growth factor to lure growing axons toward their natural targets, thus leading to a progressive recovery of walking three or four weeks after SCI.
An interesting hypothesis that shapes the Anderson Squair study is that the glial scar, long thought to be the basic inhibitory villain of regeneration (e.g., central to the ReNetX and NervGen clinical trials), doesn’t really matter. Anderson, with Sofroniew, published work in 2016 showing that, contrary to prevailing dogma, astrocyte scar formation actually aids rather than prevents axon regeneration. In the most recent paper, certain switched-on axons were coaxed right through the scar.
I asked Anderson what’s next:
We have begun a project investigating the ability of our regenerative strategy to restore hand function after cervical SCI in rodent models and we have also begun scaling up this strategy to improve walking after nonhuman primate SCI. We are also investigating the requirements necessary to regenerate different supraspinal neurons that will be necessary to restore specific neurological functions.
We will continue to follow this work as these scientists and others figure out how to regenerate spinal cord nerves and eventually, one hopes, to restore the functional integrity of the cord.
Note: Mark Anderson has agreed to present this work at the 2024 U2FP Science and Advocacy Symposium, in Atlanta this coming September.