July 10, 2025
Headline Patrol: Three Headlines, Two “Breakthroughs,” All Clinical Upsides
Sam Maddox
Here are three recent headlines on SCI research, two of which are labeled as ‘breakthroughs’. Each appears to have a clinical upside, let’s dig in.
Here’s a very creditable clinical result reported in May from the University of Texas (UT) at Dallas for closed-loop vagus nerve stimulation (CLV). The trial included rehab and saw hand recovery in 19 individuals with chronic, incomplete cervical injuries. A small device is attached to the vagus nerve (near the collarbone); high intensity stim is timed with real-time specific repetitive hand task movements while playing simple video games over 36 therapy sessions.
Investigators reported significant increases in pinch force, wrist torque (e.g., ability to turn a doorknob), speed, range of motion, and overall scores on a hand function grading scale called GRASSP – including on the untrained arm.
Said the study team, “This improvement exceeds the preregistered outcome and casts doubt on the long-held notion that additional gains are not possible in people with traumatic SCI more than 1 year post-injury.”
From the group’s publication in Nature on what’s happening:
. . . . (earlier VN) studies have revealed that the combination of high-intensity rehabilitative training with real-time VNS produces synaptic plasticity in motor control networks in the cortex, subcortical structures and spinal cord to engender functional recovery that is not possible with training alone. Concurrent implementation is a key component, as decoupling the delivery of VNS by even tens of seconds from the appropriate movements fails to promote recovery, consistent with the reliance on the synaptic eligibility trace. Moreover, efficacy is dependent on hundreds of VNS–movement pairings per day for many weeks.
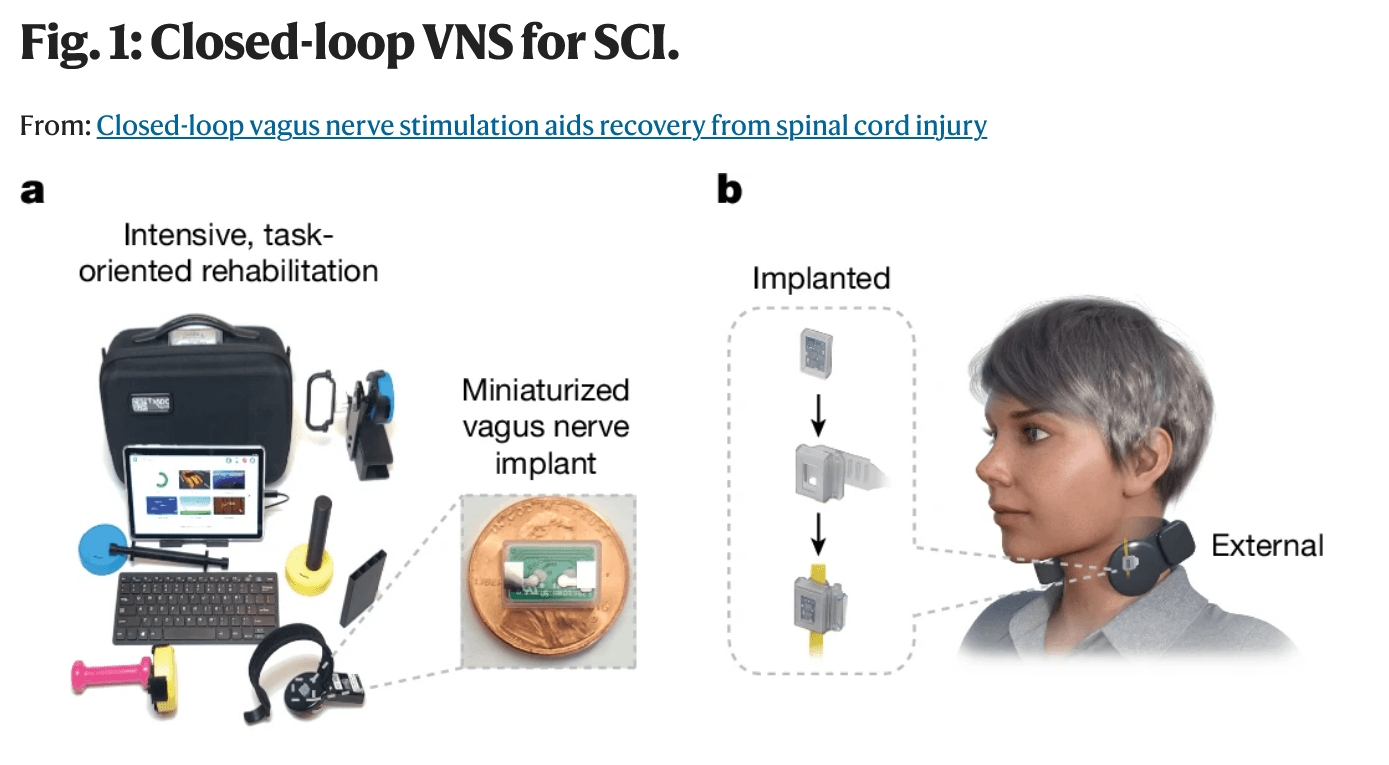
Figures 1a and 1b, from the article by Kilgard, M.P., Epperson, J.D., Adehunoluwa, E.A. et al. Closed-loop vagus nerve stimulation aids recovery from spinal cord injury. Nature (2025). https://doi.org/10.1038/s41586-025-09028-5
In 2021, the UT group got FDA approval for vagus stimulation for stroke; the stim, they said, could rewire brain function when accompanied with physical therapy.
Michael Kilgard, corresponding author for both the stroke study and the recent SCI work and professor of neuroscience in the UT School of Behavioral and Brain Sciences, explained that treating SCI is not the same:
“In stroke, people who do only therapy may get better, and adding CLV multiplies that improvement. This study is different: Therapy alone for spinal cord injury didn’t help our participants at all. It is truly groundbreaking that we’re creating a gain where there otherwise would be none.”
The positive results now permit the UT scientists to proceed with a larger pivotal trial, enrolling 70 participants at multiple sites.
Dr. Seth Hays, associate professor of bioengineering and co-author of the study, noted that while these results are promising, the path to widespread clinical availability still faces challenges.
"We still have a long road ahead. For many reasons — financial, regulatory or scientific — this could still die on the vine," he said. "But we have positioned ourselves to succeed."
Potential safety issue: Since the vagus nerve controls autonomic function, VNS could in theory influence cardiovascular function or induce autonomic dysreflexia in individuals with SCI. Kilgard’s group said they saw no changes in heart rate or blood pressure between active and sham stimulation, and no instances of autonomic dysreflexia.
Clinical/commercial potential? Seems likely. The vagus stim stroke therapy is on the market as Vivistim, from a company called MicroTransponder. Kilgard discloses a financial interest in the company.
Note: The work was sponsored by Wings for Life’s Accelerated Translational Program and the Defense Advanced Research Projects Agency Biological Technologies Office.
2. NervGen Pharma Announces Breakthrough Results in Spinal Cord Injury Treatment Trial

Shutterstock image from the article.
Much anticipated results were released in June from the Connect study testing a peptide drug, NVG-291, to promote nervous system repair in chronic cervical SCI. Good news for sponsor company NervGen: The trial met an endpoint demonstrating improved motor connectivity, showing a three-time increase in an electrical measurement of a hand muscle signal (first dorsal interosseus). A second endpoint, connectivity in a leg muscle (tibialis anterior), did not reach statistical significance.
“This is the first placebo-controlled trial of which we are aware that an investigational drug candidate has achieved statistical significance on a primary endpoint, in this case a quantitative biomarker of motor connectivity.” — Daniel Mikol, MD, Ph.D., NervGen Chief Medical Officer
The electrical signal is deemed by trial administrators as significant, but it did not convincingly translate to functional gain. The study measured grip and grasp strength using tasks such as pouring water into a cup and inserting a key into a lock and turning it 90 degrees – this so-called qualitative prehension test trended toward beneficial effect (e.g. 7 in 10 got better than those in the placebo group) but not enough to be statistically meaningful.
Two participants in the drug group could not initiate the walking test at the beginning but could at study end. This, the company notes, is likely due to the physical therapy the trial included. One person in the control group also got a big bump (1200%) in walking speed, they don’t know why.
The trial enrolled 20 patients, 10 got the drug, 10 got placebo. They all just found out which group they were in. Several have taken to social media to offer some detail. One must read these sorts of anecdotal comments with squinted eye – they’re interesting but don’t tip the empirical scale one way or the other.

On Reddit, a woman with the display name “laugh_Alotl_Axolotl” (real name Leslie Fuller, a C5 AIS C injured in 2016) described getting daily injections for 12 weeks during the trial. She found out she was on the drug. She reported that her walk speed was better, she could perform some hand dexterity tests better, and her sense of smell returned. “Want to add a big, subtle thing about being on the med was I felt like myself again! Somewhere, something turned down the noise. I had a renewed sense of wellbeing. I had a glow up!”
Was it the drug, was it the PT, was it being the center of attention for four months at the Shirley Ryan rehab center in Chicago? For her part, Leslie wants more of the drug, and says she has gotten approval to continue taking NVG-291, thinking the 12-week trial dosage may not have allowed enough time for a more robust nerve growth response.
Larry Williams, who was in the trial and who got the drug, was recently interviewed by the Blink of an Eye Podcast. He told host Louise Senft that he went from AIS C to D, walked more fluidly while tripling his 10-meter walk score, and got quite a bit of finger dexterity back. He offered that he’d never gotten any rehab for his hands so you can’t rule out the effect of therapy alone. Anyway, Larry has a Facebook page, shares what he can about his “profound experience.” “This is hope,” he says. “This gives me the foundation to keep improving.”
Leslie Fuller was also featured on a Blink podcast. She told Senft she’s convinced the drug helped her. “We [her participant group] were not ready to dance with stars in 90 days, but we regrew parts of our body. So I believe that we have not seen anything yet. I'm super excited about it.” Her message to the community: expect therapies to come along.
The June NVG-291 announcement presented only a snapshot of the trial results, which will be more fully explored and published in the coming months. The positive result gives NervGen some wind in the sails. CEO Mike Kelly told an investor’s conference that NervGen will approach the FDA soon to see what the path to regulatory approval is, not needing, he said, to wait for the subacute results to keep the trial going, to work with dose and timing, patient selection, etc.
NervGen’s stock (follow it here) actually bombed with the trial news. To the extent this reflects investor expectation, it seems they wanted to see a lot more than improved electrical signal in the drug group.
Meanwhile, NervGen is still recruiting a 20-participant sub-acute cohort (see trial here), limited to those within 20 to 90 days of injury. We anticipate those results next summer.
3. To Walk Again, 60 Minutes
60 Minutes recently featured spinal cord injury research focused on walking recovery using implanted stimulators on the spinal cord triggered by user brain waves to actuate stepping.
This “digital bridge” story is not new around my readership. U2FP and I have covered this and all of principal investigator Gregoire Courtine’s work for years, going back to his grad school days at UCLA, and of course including his high-profile science publications and his commercial developments with the device company ONWARD Medical.
60 Minutes, the most watched TV news program in the U.S., was slow to pick up the story; this piece puts new faces on work Dr. Courtine’s lab published two years ago, which generated a lot of attention because the study participants walked “naturally,” per the headline in the Nature paper (covered here).
I wasn’t planning to discuss the 15-minute 60 Minutes segment – I don’t want to be the scold who has to say here we go again, again – except that to say reporter Anderson Cooper cluelessly served up the same gee-whiz recipe: selfless scientists doing amazing things with brain chips and lifeless limbs; compelling and inspiring subjects overcoming odds to become slightly less tragic. Peak ‘paralyzed man walks’ feelgood dis-equanimity.
60 Minutes gave a huge boost to the implantable bioelectrics field. The research group deserves our kudos, it demonstrates a nifty bit of bioengineering. This also has to be great for shareholder value in the sponsor company, ONWARD Medical. But in the end, public perception is affirmed that the SCI community is fixated on walking. This is dodgy and misleading.

From the ONWARD Medical website.
Anyway, the Monday following the 60 Minutes broadcast, ONWARD Medical CEO Dave Marver dropped me a note suggesting a Headline Patrol, but not on the main “To Walk Again” segment. He wanted to call attention to a 60 Minutes Overtime sidebar segment, which acknowledges that walking is cool but not the only thing his company thinks about.
I told Marver, sure, I’d include the Overtime segment, but I’d need him to set it up, perhaps responding to critical feedback the likes of what Amy Van Dyken, the former Olympic swim champion who now lives with an SCI, dished out on her Instagram after seeing the piece: “That was rude,” she noted, “pretty (f-bombage) rude.”
Slight digression: Here’s what Amy had to say after someone asked, gee don’t you want science to help you wheelchair people?
ASK US!! I am so thankful for research being done on spinal cord injuries. What @60minutes showed is NOT a cure, and not what most of us want back. Sure walking would be amazing, but ask most of us and we want to know when we have to pee, when we have to poop, and we want to feel our sexy bits!!! That’s what they should be looking into FIRST! I feel researchers are getting their grant $$ and trying to make us walk, because they THINK that’s what we want. Just ask us. If you really want to help us…ASK US!!
Ed. note: Marver was recently interviewed for the U2FP CureCast podcast; he talks about the 60 Minutes story. This segment will post soon.
Anyway, here’s my short exchange with Marver, the ONWARD Medical CEO, who was interviewed in the Overtime segment.
Brief summary: Anderson Cooper and his producers came to do a story on BCI. While here, we explained the importance of other recovery targets (bladder, bowel, sexual function, blood pressure). I covered much of this in my interview, which unfortunately did not make the main segment. Anderson cared enough about telling this story that they produced a second segment for 60 Minutes Overtime, which gets good exposure on YouTube and the CBS website.
Thanks for flagging the post by Amy Van Dyken. I can understand her frustration. She obviously did not see the Overtime segment. We need to do a better job communicating that we understand the priorities of the community and we are doing our best to advocate for them when we engage with media. More importantly, we are investing in the development of therapies that align with the community’s priorities: Our first therapy addresses hand function and our second will address blood pressure instability.
Marver, in the Overtime segment, was slow-pitched a question about where ONWARD would like to be in 10 years. He said it would be great if people with SCI could have an informed discussion with their doctors about what needs to be fixed, and pick from a sort of stimulation therapy menu. That might include hand function, bowel health, incontinence, sexual function, pain. And if it includes walking, you can be sure you’ll hear about that first.
Conclusion
Two electrical stimulation studies, one biologic, all moving through clinical trials. Vagus nerve stim is probably closest to being an approvable intervention, since it’s already been cleared for market in stroke. ONWARD, which has a skin surface stim device on the market now, and which is on track to get its implanted epistim unit approved for blood pressure management, maybe next year, is not close to making brain chip-epidural stim a real option. As for NervGen, the 10 patient chronic SCI cohort showed encouraging electrical activity below the level of injury in certain muscle groups. Too early to say the jury is out – the case hasn’t really been fully tried and there’s a lot of hearsay floating about. But we’ll keep an eye on it, and so will Reddit.
PS - These potential treatments don’t appear out of thin air. Years ahead of clinical trials, the scientific discoveries alone require smart, targeted funding. You might remember that the idea which eventually contributed heavily to ONWARD’s ARC-EX device came from a small state-based grant in Washington state. U2FP’s CAN led the charge to get that funding approved. We’re on the verge of getting another bill passed in Wisconsin - if you can, give these Senators a call and tell them to support SB99.