Dec 30, 2023
2023 SCI Literature in Review
Sam Maddox
Here’s a look back at spinal cord injury research studies published in 2023. I selected a few of my favorites from this past year. Then, to make this more expansive, I invited members of the U2FP Scientific Advisory Board (SAB) to pick a paper or two that caught their interest. This compilation is not ranked or scored, it’s not meant to be comprehensive or all-inclusive.
I’ll go first, followed by the SAB – Moses Chao, Paul Lu, John Houle and Keith Tansey. l also added a paper picked by U2FP Board Chair Sasha Rabchevsky.
Bran Stark (left) played by Isaac Hempstead Wright and Samwell Tarley (right) played by John Bradley in the HBO series Game of Thrones.
1. Regeneration redux. “Recovery of walking after paralysis by regenerating characterized neurons to their natural target region,” was also chosen by Paul and John (see their comments below).
This work is a multidisciplinary, combinatorial, and collaborative effort between UCLA, the Swiss Federal Institute of Technology, and Harvard University, lead authors Jordan Squair and Mark Anderson. It’s innovative, ambitious, audacious – for an acute SCI study using mice, it makes a giant hypothetical leap toward reengineering the damaged cord to restore function.
This group worked out the axon regeneration part a few years ago, but the growing nerves didn’t connect functionally. Said senior author Michael Sofroniew, MD, PhD, at the David Geffen School of Medicine at UCLA, “It highlights the necessity of not only regenerating axons across lesions but also of actively guiding them to reach their natural target regions to achieve meaningful neurological restoration."
After finding the molecular and anatomical properties of the specific spinal cord nerves (Vsx2) that naturally recover, the scientists induced them to regenerate by reactivating dormant genetic growth programs last used during development. They then added a support matrix so the axons had something to grow on across the lesion core. To steer the axons’ growth, the scientists applied chemical signals to attract and guide them across the spinal cord injury site to form a connection below. This led to improvements in walking ability, a progressive recovery occurring three to four to weeks after SCI.
Lots of work to do here (let’s see this in a chronic, larger animal model!) to get near translation but this one fires the imagination and restores a measure of faith in the notion that SCI regeneration is possible, with the right recipe, in the right hands.
2. Shout out to SpineX, the L.A. based company started by neuromod maven Reggie Edgerton. In a paper called “Combining spinal neuromodulation and activity based neurorehabilitation therapy improves sensorimotor function in cerebral palsy,” the company reported that noninvasive Estim (they call it SCiP) significantly benefited six kids with cerebral palsy. From the looks of videos from the company, this use of Estim is making a real impact on kids with CP.
Said Jessica Oldham, whose nine-year old daughter Annika lives with CP:
Our experience with SCiP has been a true game-changer for our child. When she started her first series of therapy sessions, we saw almost immediate results. She went from using a walker or crutches almost exclusively to standing up straighter and using just one crutch/walking stick. She has also begun to walk independently in our home.
3. Exosomal upside: This is a review paper, which highlights the field and not only a particular study. (Note: partly funded by our friends at the Bryon Riesch Foundation.)
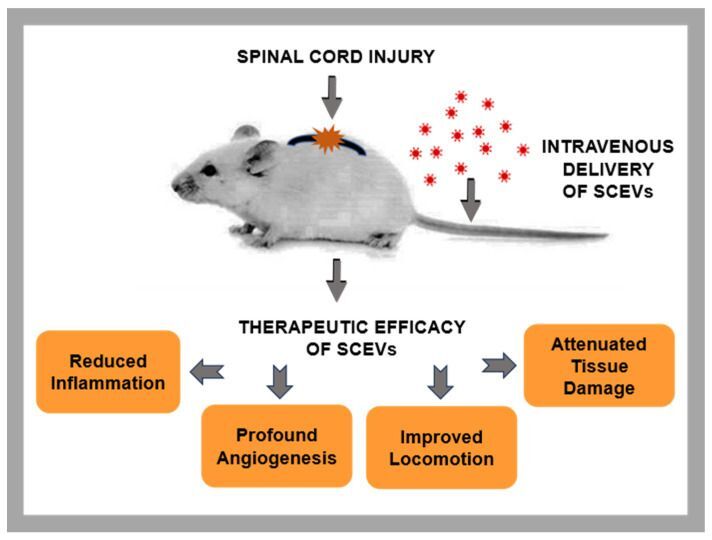
Scheme 1. Therapeutic benefits of administering SCEVs in rodent models of SCI. Image credit:International Journal of Molecular Sciences, published online 2023 Dec 10.
In this paper, “Schwann Cell-Derived Exosomal Vesicles: A Promising Therapy for the Injured Spinal Cord,” a group led by Damien Pearse, PhD, at the Miami Project reports on the upside for a biologic approach to SCI repair that doesn’t use whole cells. Exosomes are small enough to cross into the CNS past the blood brain barrier, appear to be safe and stable, can be targeted, can be engineered to carry drugs or biologic agents, and offer advantages to cell-based therapies. This paper specifically considers Schwann cell-derived exosomal vesicles as an approach to SCI protection and repair.
From the paper:
Exosomes are nanoscale-sized membrane vesicles released by cells into their extracellular milieu. Within these nanovesicles reside a multitude of bioactive molecules, which orchestrate essential biological processes, including cell differentiation, proliferation, and survival, in the recipient cells. These bioactive properties of exosomes render them a promising choice for therapeutic use in the realm of tissue regeneration and repair.
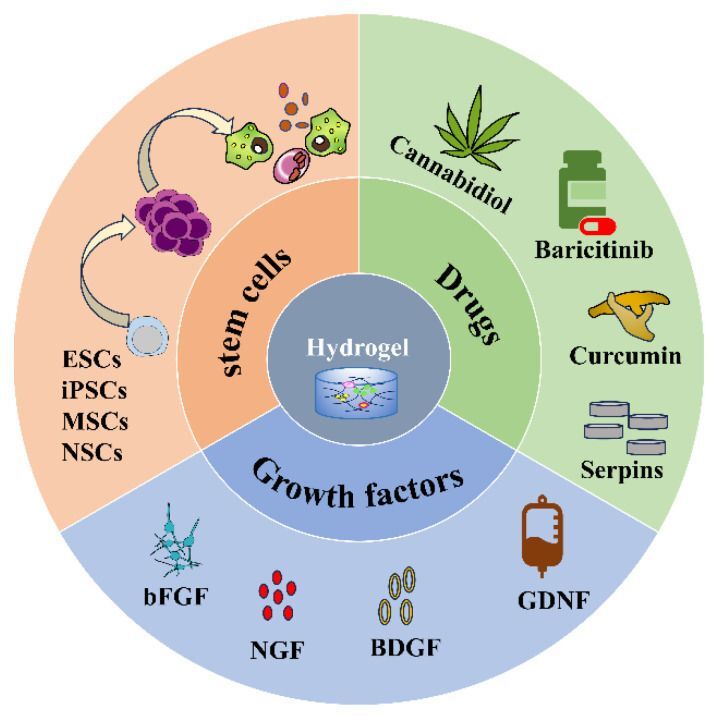
Figure 2. Hydrogel loaded with stem cells, drugs, and growth factors. Image credit: Gels, published online 2023 Nov 16.
4. Gels gain gravitas. “Application of Injectable Hydrogels as Delivery Systems in Spinal Cord Injury” is a review paper from China recognizing the emerging potential of injectable hydrogels, which are engineered 3-D polymeric materials that can be tuned for structural and functional design. Hydrogels can be used as delivery systems.
From the paper:
Given their good biocompatibility, biodegradability, and low immunogenicity, injectable hydrogels can be used as delivery systems to achieve controlled release of drugs and other substances (cells and proteins, etc.), offering new hope for SCI repair. Hydrogels are anti-inflammatory, antioxidant, anti-apoptosis, and pro-neurogenesis.
Another Chinese review, “Recent advance in bioactive hydrogels for repairing spinal cord injury: material design, biofunctional regulation, and applications,” shows how gels promote SCI repair.
From the paper:
We demonstrate various methods and techniques for the fabrication of bioactive hydrogels with the biological components such as DNA, proteins, peptides, biomass polysaccharides, and biopolymers to obtain unique biological properties of hydrogels, including the cell biocompatibility, self-healing, antibacterial activity, injectability, bio-adhesion, bio-degradation, and other multi-functions for repairing SCI.
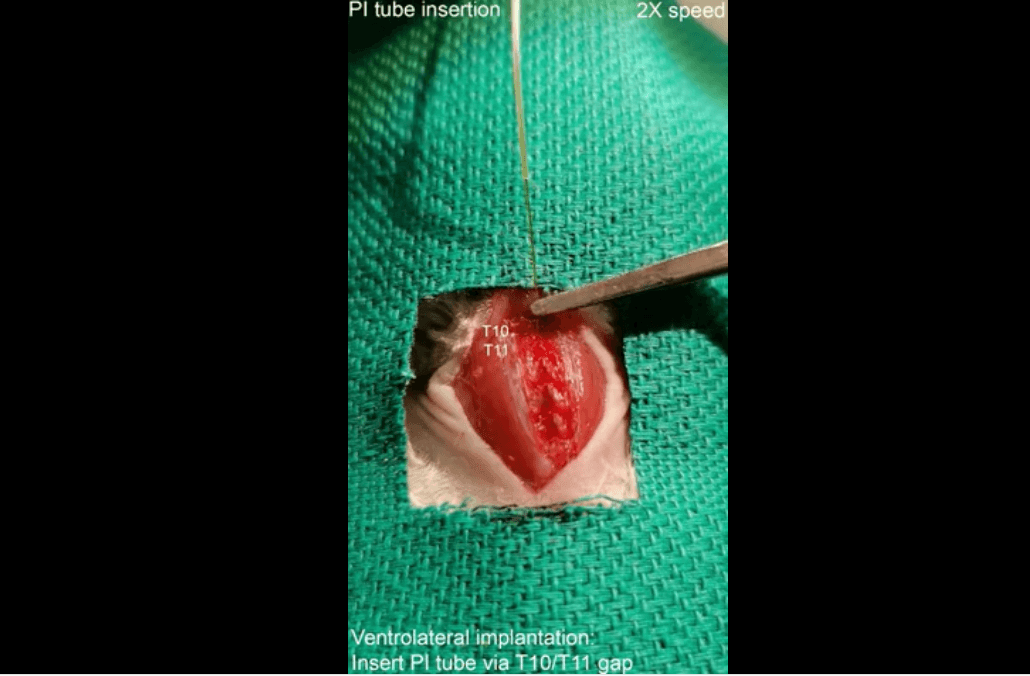
Figure 1. Implantation procedures (dorsal and ventrolateral). Image credit: Nano Letters, published online 2023, Jul 1.
5. Nano bioelectronics. This is a recent study from a group at Johns Hopkins that broadens the potential lane for miniature nanotech stim devices. “Injectable Ventral Spinal Stimulator Evokes Programmable and Biomimetic Hindlimb Motion” describes a tiny, flexible, minimally invasive injectable spinal cord stim device that on paper at least is more precise and has major advantages over implanted epidural stim units for fine-tuned activation of lower limbs post paralysis.
Said lead author Dinchang Lin, a researcher at Johns Hopkins Institute for NanoBioTechnology:
Applying this new technology in a mouse model, we evoked leg motions using an electric current nearly two orders of magnitude lower than that used in traditional dorsal stimulation. Our stimulator not only enabled a broader range of motions but also allowed us to program the electrode array's stimulation pattern, which resulted in more intricate and natural leg movements reminiscent of stepping, kicking, and waving.
Ed. note: principal investigator for this study is nano tech luminary Charles M. Lieber, the former Chair of Harvard’s Chemistry and Chemical Biology Departments – former because he was convicted, fined and jailed (for two days) in 2023 for lying to federal authorities about his affiliation with a Chinese tech recruitment program and for evading income tax.
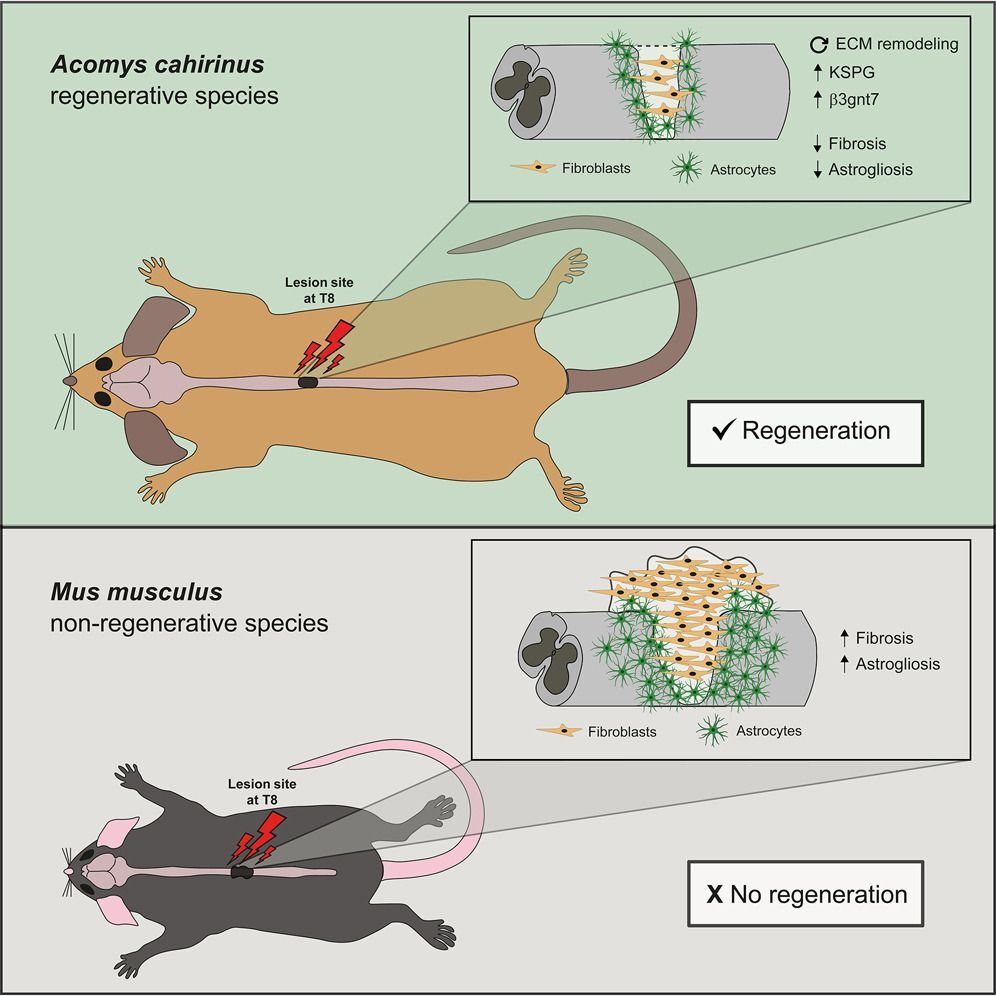
6. Meet spiny mouse, preclinical rockstar. What’s this little guy got going that all of us other mammals don’t? Wouldn’t we like to know. It’s got a superpower, same as axolotls, same as zebrafish: the ability to self-repair, including a busted spinal cord.
Here is a 2022 paper I heard about this year, featuring this uniquely amazing mammalian creature. “Rewired glycosylation activity promotes scarless regeneration and functional recovery in spiny mice after complete spinal cord transection” is from a group in Portugal led by Monica Sousa.
Says the paper:
… we reveal that Acomys (spiny mouse) is able to do what no other adult mammal can: spontaneously regenerate its spinal cord after complete transection with fast restoration of function. Our data show that Acomys assembles a pro-regenerative environment at the injury site with rewired glycosyltransferase activity, dictating a specific proteoglycan signature. Our results disclose the spiny mouse as a valuable model to explore the molecular mechanisms enabling robust functional axon regeneration in the adult mammalian CNS.
7. Stim plus stem, better together. “Electrical stimulation affects the differentiation of transplanted regionally specific human spinal neural progenitor cells (sNPCs) after chronic spinal cord injury” is a new paper from Ann Parr’s group at the University of Minnesota. Combo therapies are likely to be foundational for SCI treatment. Here, we appreciate seeing the words “chronic” and “human cells” in a preclinical SCI study. Says the paper, “. . . electrical stimulation promoted cellular integration and influenced the fate of human induced pluripotent stem cell-derived sNPCs transplanted into the injured spinal cord.”
SAB Selections

John Houle is a professor in the Department of Neurobiology & Anatomy at Drexel University College of Medicine, and director of the Spinal Cord Research Center.
1. Neural stem cell therapies for spinal cord injury repair: an update on recent preclinical and clinical advances. Seyed Mojtaba Hosseini, Ben Borys, Soheila-Karimi Abdolrezaee. Brain, 2023 Nov 17.
There is significant interest in the potential use of neural precursor cell-based therapies for spinal cord injury repair, though many challenges have limited the benefits realized in preclinical studies to date. This review provides an update on current clinical trials and new technologies that may begin to push this field forward.
2. Neuromodulation Through Spinal Cord Stimulation Restores Ability to Voluntarily Cycle After Motor Complete Paraplegia. Hoover C, Schuerger W, Balser D, McCracken P, Murray TA, Morse L, Parr A, Samadani U, Netoff TI, Darrow DP. Journal of Neurotrauma, 2023 Mar 22.
3. Epidural stimulation restores muscle synergies by modulating neural drives in participants with sensorimotor complete spinal cord injuries. Singh RE, Ahmadi A, Parr AM, Samadani U, Krassioukov AV, Netoff TI, Darrow DP. Journal of NeuroEngineering and Rehabilitation, 2023 May 3.
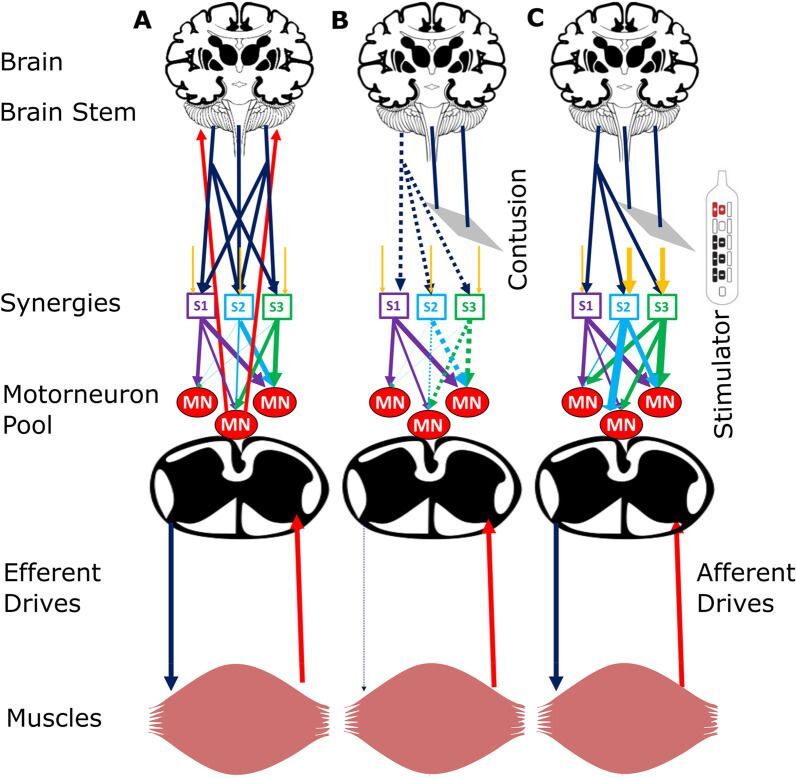
Figure 10. Schematic of hypothetical neural mechanisms. Image credit: Journal of NeuroEngineering and Rehabilitation, published online 2023 May 3.
Studies 2 and 3 are from investigators at the University of Minnesota. This group demonstrated the ability to affect specific voluntary motor function after epidural stimulation combined with motorized cycling training regardless of the time post injury. This is a significant advance towards establishing a pattern of relearning in SCI individuals for a specific task that can be performed at home. The results in the third paper demonstrate the return of complex muscle activity with continued epidural stimulation, which leads to improved coordination between muscle groups. Together these studies advance our knowledge of the beneficial effects of epidural stimulation and the importance of seeking approaches that can be applied to a larger proportion of the SCI population.
4. Recovery of walking after paralysis by regenerating characterized neurons to their natural target region. Squair JW, Milano M, de Coucy A, Gautier M, Skinnider MA, James ND, Cho N, Lasne A, Kathe C, Hutson TH, Ceto S, Baud L, Galan K, Aureli V, Laskaratos A, Barraud Q, Deming TJ, Kohman RE, Schneider BL, He Z, Bloch J, Sofroniew MV, Courtine G, Anderson MA. Science, 2023 Sep 22.
In this study by a group of leading investigators, experiments questioned the level of specificity necessary to achieve functional recovery attributable to regenerating axons, as to whether they needed to be directed to their original targets or whether they simply needed to establish a new connection below the level of injury. Using sophisticated targeting and growth promoting techniques this group demonstrated that reactivation of axon growth and subsequent guidance from a combination of functionally relevant subpopulations of neurons was necessary to achieve meaningful repair (i.e. walking) in injured mice.
5. Recovery of Forearm and Fine Digit Function After Chronic Spinal Cord Injury by Simultaneous Blockade of Inhibitory Matrix Chondroitin Sulfate Proteoglycan Production and the Receptor PTPσ. Milton AJ, Kwok JCF, McClellan J, Randall SG, Lathia JD, Warren PM, Silver DJ, Silver J. Journal of Neurotrauma, 2023 Dec.
The lab of Jerry Silver used a combination approach to affect the regenerative and immune system of chronically injured rats to achieve recovery of walking and precise movement of digits. Animals up to 1.5 years post injury were treated to promote functional synaptic plasticity.
(Ed. note: the receptor PTPσ peptide used in this paper is being tested in the NervGen clinical trial, currently enrolling.)

Paul Lu is a neural stem cell scientist at the University of California, San Diego and member of Mark Tuszynski’s lab.
(Ed. note: This is a discussion of the aforementioned Anderson study, "Recovery of walking after paralysis by regenerating characterized neurons to their natural target region," reporting recovery of walking after SCI by targeting spinal cord interneurons and manipulating multiple genes.)
An important study from Mark Anderson’s group at the Swiss Federal Institute of Technology was published in September 2023 in the high impact journal, Science. The study identifies a spinal interneuron population called Vsx2 that are essential to restore walking after low thoracic spinal cord injury in mice.
They targeted this special Vsx2 population for regeneration across a complete crushed SCI site and then into their natural spinal cord targets, L2, an area of the spinal cord that controls walking. The investigators used a combinatory approach: First, they activated the intrinsic growth capacity of Vsx2 neurons by over-expressing three genes (osteopontin, insulin-like growth factor 1, and ciliary-derived neurotrophic factor, CNTF) above the injury at T10; second, they created a T13 SCI just above the lumbar area and provided growth–supportive, permissive substrates within the lesion by delivery of fibroblast growth factor 2 (FGF2) and epidermal growth factor (EGF) (both proteins); third, they delivered glial-derived neurotrophic factor (GDNF) by lentivirus as a chemoattractive agent below the injury at L2 to attract regenerated axons toward this region.
Anatomical analysis reviewed that Vsx2 derived axons from above SCI site regenerated into lesion site at T13 and then toward their natural target region in the lumbar spinal cord (L2). This regrowth coincided with leg movement recovery starting around 3-4 weeks after SCI. They reported that their regeneration strategy led to substantial recovery of walking after complete SCI.
The authors manipulated multiple genes above, within, and below the injury site, to promote this special population of spinal cord interneurons to generate into and beyond SCI, including four over-expression genes by viral vectors, (Osteopontin, IGF-1, CNTF, and GDNF), and two proteins (FGF2, EGF) within SCI site. Many of these genes have been shown to have effect on axon regeneration, but simultaneous delivery of four genes by viral vectors and two proteins is rarely reported in the SCI research field. These results indicate that a complex disorder, such as SCI, requires a complex and combinatorial approach as a treatment.
This study achieves not only great anatomical regeneration after complete SCI but also functional improvement. However, there are several challenges before translation to clinical treatment is realistic.
First, over-expression of GDNF and other genes may have undesired side effects. Short-term or regulable delivery of genes by gene therapies could solve these problems.
Second, this study used a very narrow lesion size, about 200 µm (Fig. 3B), while the mouse spinal cord can be 10x bigger in size (2mm in diameter). Regenerated axons could easily cross this narrow lesion size. Moreover, human SCIs usually contain cavities; simple FGF2 and EGF treatment may not generate enough cells to fill up these cavities to support axons to grow. Exogenous cell transplantation may be needed to fill up the cavities and bridge the injured spinal cord.
Third, the SCI lesion here is just above the lumbar spinal cord and regenerated axons can reach their natural targets by a short-distance of growth. However, clinical SCI occurs in all levels and long-distance regeneration is required to gain functional recovery in the majority of cases.
Lastly, although this study showed robust axon regeneration beyond the SCI site, most of these regenerated axons could be branches from only a few regenerated axons. Indeed, whether these few regenerated axons could elicit functional recovery is questionable.

Keith Tansey is a physician/scientist at the University of Mississippi, Jackson VA and Methodist Rehabilitation Center.
Says lead author Tansey:
The take home message is that there are different responses to neuromodulation in different groups of patients, and between different parts of a motor task like stepping, and that the most impactful stimulation settings varied across these groups with effects ranging from diminishing or inappropriate motor output to augmenting appropriate motor output. Bottom line, we show that this therapeutic approach will definitely not be a "one size fits all" in terms of the effects it has on the injured spinal cord or on the motor tasks it is attempting to execute.

Moses Chao is a neuroscientist and university professor at NYU Langone Health Medical Center.
"Confronting the loss of trophic support," Moses Chao, principal investigator. This paper asks the question, how do neurons survive in the absence of trophic support, such as NGF and BDNF? It’s a complicated biologic mystery but many nerve cells do adapt and wean from trophic support.
From the paper:
We developed cellular assays to assess trophic factor dependency for sympathetic and hippocampal neurons and identified factors that rescue neurons in the absence of trophic support. They include enhanced expression of a subunit of the NGF receptor (Neurotrophin Receptor Homolog, NRH) in sympathetic neurons and an increase of the expression of the glucocorticoid receptor in hippocampal neurons. The results are significant since levels and activity of trophic factors are responsible for many neuropsychiatric conditions. Resistance of neurons to trophic factor deprivation may be relevant to the underlying basis of longevity, as well as an important element in preventing neurodegeneration.
Also, kudos to Chao on the publication of a new book, Periphery: How Your Nervous System Predicts and Protects against Disease.
From the promo blurb:
As Moses Chao argues...many neurological conditions emerge not in the brain but rather within the peripheral nervous system, in the dense network of nerves that wrap around the gastrointestinal tract. What's more, dysfunctions of the peripheral nervous system can signal the onset of disease decades before symptoms like tremor or memory loss occur. Fortunately, unlike nerves in the brain and spinal cord, peripheral nerves can heal and regenerate in response to injury and aging. The therapeutic implications are remarkable. Chao shows how, with a better understanding of the peripheral nervous system, we could not only predict and treat neurological diseases long before their onset, but possibly prevent them altogether.

Sasha Rabchevsky, scientist and Professor at the University of Kentucky College of Medicine and member of the UK Spinal Cord & Brain Injury Research Center. He is board chair for U2FP.
"Perspectives on Data Sharing in Persons With Spinal Cord Injury," lead authors John Kramer and Freda Warner from the International Collaboration on Repair Discoveries (ICORD), Blusson Spinal Cord Centre, University of British Columbia. Rabchevsky is co-author.
This study reports results of a survey of 232 people living with SCI. Would they be willing to share data if they were involved in a clinical study? Yes, in short, they are pretty OK with that.
From the paper:
Open data sharing of clinical research aims to improve transparency and support novel scientific discoveries. There are also risks, including participant identification and the potential for stigmatization. The perspectives of persons participating in research are needed to inform open data-sharing policies. The aim of the current study was to determine perspectives on data sharing in persons with spinal cord injury, including risks and benefits, and types of data people are most willing to share.
The End/the Beginning
This selection of 2023 papers are almost all animal studies, and there are no certainties any will lead to a treatment. But they do illustrate that the pursuit of restoration of function post spinal cord injury is a field full of new ideas, new materials, and new therapeutic concoctions. There seems to be a willingness to veer outside the lanes that define the engineering and biologic disciplines. There seems to be a sense of possibility. Of risk. Maybe even urgency. Let’s keep the pedal down in the new year.
Stay curious.