May 5, 2025
Headline Patrol: Stem Cells Old and New
Sam Maddox
This is about stem cells and their clinical potential for spinal cord injury recovery. You don’t hear as much about these cells as we once did, but as you will see, active human trials are still being conducted.
I started out writing about a small, soon-to-recruit clinical trial injecting stem cell-derived cells into two groups of people with spinal cord injury: subacutes (3 to 6 weeks) and chronics. The subacute cohort, of course, doesn’t have the slightest clue any of this is coming. For people with existing SCI, though, the news is rare, and welcome.
This trial continues a story very familiar to anyone following SCI research in recent years. The cell line, oligodendrocyte precursor cells (OPCs) started as two-day-old embryos in the late 1990s, went to the lab, picked up some political baggage along the way and later broke new ground in clinical regenerative medicine. They’re still being propagated, still experimental, but still relevant.
Headline Alert
As I was pulling the OPC update together, a couple of stem cell related headlines popped up. Let’s start there.
The first one appears in the influential journal Nature: “Paralysed man stands again after receiving ‘reprogrammed’ stem cells.”
The other features thin coverage but with a click-bait verb choice: “Paralyzed Man Standing, Learning to Walk Again After Injection of Hacked Stem Cells,” from an internet site called Futurism.
Headline and image from a futurism.com article by Victor Tangerman (image by Getty / Futurism).
These articles refer to a Japanese study of four people with subacute (24 days) SCI, all AIS A (complete injuries) who were injected with a type of stem cell that is genetically reverse-programmed from an adult donor cell (e.g. skin or blood cell) to assume the robust characteristics of a foundational pluripotent stem cell.
The cells are called induced pluripotent stem cells, iPSC, discovered by Japanese scientist Shinya Yamanaka in 2007 (he won the Nobel Prize for this work in 2012). iPS cells can then be redirected in the lab to become any other cell type; in this case, the cells became neural precursors.
The newest standing man trial was run by Hideyuki Okano at Keio University in Tokyo. We don’t have much to go on regarding the study – this result comes from news reporting and has not been peer reviewed or published, although the study protocols were, in 2021.
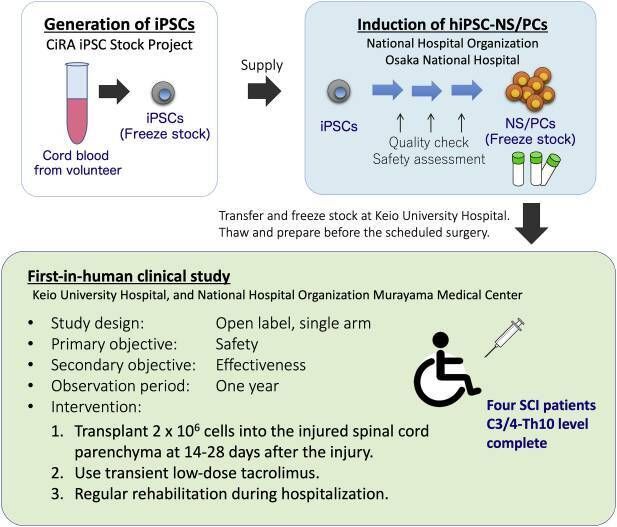
Fig. 5 from First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: Study protocol. Regen Ther. 2021 Sep 7;18:321–333. doi: https://doi.org/10.1016/j.reth.2021.08.005
What’s been reported is that one guy got three AIS levels of improvement, can stand and is training to walk. Another gained two AIS levels and can move arms and legs. Two didn’t show improvement.
Three levels, said Okano, is dramatic, and he is right. But he is not claiming that recovery was due to the treatment; with such a small sample one cannot rule out spontaneous recovery, which occurs to some degree in all cases of SCI.
In the protocol paper, Okano’s group described their process. They would have preferred autologous cells (personalized from the patient's own body) but the processing cost for one-off cell batching is prohibitive. So iPS cells were used, and since they will be seen as invaders, the body will try to reject them. Therefore, the Japan study applied an immune-system suppression drug, (the same drug, tacrolimus, used in the OPC trials, which you’ll learn more about below).
What do they suspect the iPS cells will do once transplanted? The working theory:
1) remodeling the injury environment by trophic factors secreted by transplanted cells and/or their progeny (functional improvement of residual cells, promotion of regeneration of the spinal cord, suppression of inflammation, etc.).
2) repair of neural circuits by transplanted cells (replacement of lost or damaged nerve cells).
3) remyelination by oligodendrocytes differentiated from transplanted cells.
What about rehab? Only standard rehab will be used (up to 3 hours a day for 60 days) with no extra activity-based training:
The use of special equipment such as gait-assist robots as part of rehabilitation is unacceptable considering that therapeutic effects of these equipment and the transplanted cells could be mixed-up. Using this standard, we will attempt to achieve a uniform rehabilitation program for all patients, using data from patients treated in facilities specializing in SCI therapy as a historical control.
And the tumor problem? This is a real concern. Per the protocol paper, “. . . the risk of tumorigenicity is a key concern in the transplantation of cells derived from pluripotent sources.” Okano notes that tumor formation will be closely monitored in human trials. In a preclinical study with an animal model, he reported that transplanted cells did expand (they called it “abnormal overgrowth”) into the spinal column and caused lower limb paralysis. Yikes.
And the cell dosage? Okano’s trial transplanted 2 million cells, the same number Geron started with in 2010. Per the protocols:
Although 2 million cells in our trial might show some effectiveness, dose escalation may induce better functional recovery. Therefore, after confirming the safety issue, a dose-escalation study is planned, similar to Asterias' [e.g. Geron] trial using human ESC-derived OPCs.
We will watch for the published paper on iPS from Okano’s group. No doubt they’d like to be sure there are no abnormal overgrowths forming in the patients, and they’d like to know why the cells worked so well in one and not at all in two others.
U2FP’s Headline Patrol is made possible by
the generous support of Bob Yant and Ann Kenowsky.
Consider joining them by making a donation today.
Donate Now
Back to the upcoming OPC trial continuation
This is about embryonic stem cells that were steered toward becoming a type of nerve support cell called an oligodendrocyte precursor cell (OPC)
Twenty years ago this May these same cells were reported by Hans Keirstead at UC Irvine to have restored function in lab animals – raising great expectations, leading to the formation of the California Institute for Regenerative Medicine (CIRM), the stem cell agency, and to a clinical trial by the company Geron, beginning in 2010.

A year later Geron bailed on the OPC trial after just five patients had been injected with the cells. The company passed things on to Asterias, which dosed 25 more participants over the next few years before handing off the cells again. The OPCs are now at home with San Diego area biotech Lineage Cell Therapeutics, the small biotech backing the new trial. L:ineage says they have purified the cell line and prepare them as an off-the-shelf product.
(If you really want the deep dive, see A short history of oligodendrocyte precursor cells (OPC) at the end of this article).
Study detail: The official title of the new Lineage trial is “Delivery of Oligodendrocyte Progenitor Cells for Spinal Cord Injury: Evaluation of a Novel Device (DOSED).”
Lineage will use OPCs to test a new cell injection device during implantation surgery. This rig does not require surgeons to stop a patient’s breathing in order to keep them perfectly still, which has been the case up to now. Instead, it moves with the body. This should allow a faster cell transfer, and per Lineage, could reduce the complexity, risk, and variability of the injections.
Note: Lineage Cell Therapeutics was a sponsor and presenter at the 2024 U2FP Science and Advocacy Symposium (watch Vice President Michael Lauw’s presentation here). We appreciate their support for our advocacy.
The DOSED trial includes:
- Participants with C-4 to T-10 injuries, complete SCI (AIS A) or sensory incomplete, SCI (AIS B).
- Enrolling 3-5 participants with subacute injuries (21 to 42 days post-injury) and 3-5 participants with chronic injuries (1 to 5 years post-injury).
- A single injection of 10 million OPC cells will be delivered to the site of damaged spinal tissue.
- Participants will be temporarily immunosuppressed, and will be monitored for up to 10 years.
The inclusion of chronic participants is nice to see, since so many trials enroll only newer injuries, but recovery in older injuries is pretty speculative and not predicted by preclinical studies. Indeed, Keirstead noted in 2005:
Locomotor ability significantly improved beyond controls only in those animals transplanted 1 week after SCI that contained robust remyelination. Animals transplanted 10 months after SCI demonstrated no remyelination and no significant improvement in locomotor ability.
Lineage CEO Brian Culley was asked about including chronic injury on a recent investors call with analysts who follow the company’s stock. He said the FDA encouraged his team to include long term injuries in the new trial.
Culley thinks plasticity and regenerative capacity of the chronic cord may be possible, despite what is known about scarring in the injury area. The inclusion of chronics is low risk and low cost, so why not.
Lineage is of course not the first to dose chronics with stem cells – especially if you count the rogue stem cell clinics that work outside of the regulatory realm. Here are three legit trials (two planning to continue).
- California based Stem Cells, Inc. sponsored a clinical trial beginning in Zurich in 2013, using fetal nerve tissue-derived cells in chronic cervical SCI. In 2015 the company reported some very enthusiastic results, including participants getting back meaningful hand function. Alas, in 2016, the company suddenly shut it all down and folded up.
- Another small chronic SCI trial was done at UC San Diego (also partly funded by CIRM with sponsorship of the biotech Neuralstem, another cell company that no longer exists). This trial enrolled four paraplegics to receive human-spinal-cord-derived neural stem cells. Long term results were published last December: the procedure appears safe; two patients showed increased motor and sensory scores, two had pain relief. The plan is to continue the trial,
- In 2020 the Mayo Clinic shared results of a small trial (n=10) called CELLTOP, using fat-cell derived mesenchymal stem cells injected intrathecally (into the spinal canal). Some showed recovery, some didn’t. One participant was deemed a “superresponder,” recovering significant mobility. We covered this in 2019; the study lead, neurosurgeon Mohamad Bydon, was on the U2FP CureCast podcast) in 2020 and appeared at U2FP’s annual Science and Advocacy meeting later that year. He speculates that the cells may reduce glial scarring, promote axonal regeneration via secretion of growth factors, or morph into neural elements. CELLTOP Part II is currently recruiting 40 more participants.
Despite a lack of clearly established mechanism of action, there are obvious advantages to working with people living with chronic SCI: patient recruitment should be easier; any potential change in function can be measured against a stable baseline, unlike in subacute, where recovery may happen on its own. From the accounting side, chronic SCI is a huge unmet need and a much better business opportunity than the acute market.
Do OP Cells Do Anything?
Good question. In the original animal studies of OPCs, three potentially reparative actions were reported: production of nerve growth factors, stimulation of blood flow, and remyelination of damaged axons – leading to improved hindlimb and forelimb function, reduced formation of spinal cord cavities, and preservation of myelinated axons at the injury site.
In people, it’s not possible to open them up to prove remyelination or specific cellular repairs. Observing the 30 participants to get OPCs so far, no adverse effects or weird growths have been reported. The cells grafted well into the cord and MRI revealed reduced cavitation.
Efficacy? Many participants got better than expected when compared to what’s called a historical control, or similar injury profiles from non-treated people.
Reporting in 2019, Asterias said that at 12 months post OPC implant, 95 percent (21/22) of SCIStar subjects recovered at least one motor level on at least one side, compared to 68 percent expected in historic controls
Also at 12 months, 32 percent treated with OPCs (7/22) recovered two or more motor levels on at least one side, that’s a little bit better than the 26 percent recovery expected.
Two level motor improvement is significant. Said the company press office:
“Patients with severe spinal cord injuries that show two motor levels of improvement on at least one side may regain the ability to perform daily activities such as feeding, dressing and bathing, which significantly reduces the overall level of daily assistance needed for the patient and associated healthcare costs.”
Anecdotally, both the company and CIRM continue to highlight individual participants whose SCI trajectory suggests OPC impact:
- The Lineage homepage background video shows two OPC trial participants, Kris Boesen and Lucas Lindner. Both have cervical SCI, both reported recovery, Lindner enough to throw out the first pitch at a 2017 Milwaukee Brewers game.
- Also on the home page is a link to a 2025 CNN segment on Jake Javier, described as “a participant in a trial using a stem cell based treatment that’s allowed him to regain the smallest movement in his hand, but enabling him to drive, type and grasp.”
From CIRM, more on Jake, who appeared on stage at the first SCI Investors Conference in 2023, sponsored by Lineage: He’s “a living testament to the power of medical innovation and personal resilience.”

Jake Javier (center) speaking a the First Annual Spinal Cord Injury Investor Symposium
Ready for the Clinic?
So, how close are OPCs, or any other cell therapy, to being an approved cell therapy for SCI?
Not close, at least not in spinal cord injury. The OPC sample size, for example, does not yet have the necessary statistical power to breach the regulatory fortress. This new OPC trial (n=10) won’t do much to speed the time frame. What would it take, 100, 200 individuals who improved one AIS level with OPC treatment? Is one level enough? Improving two levels might get things moving but that’s a tough ask. Moreover, as long as effect is measured against historical controls, will that ever be convincing enough? There may be too many variables between individuals to build solid, predictable data.
A short history of oligodendrocyte precursor cells (OPC)
Oligodendrocyte precursor cells (OPC) are quite familiar to anyone who paid even a little bit of attention to SCI research over the last 20 years. They are often called stem cells, which would mean they could become any cell in the body. Although they are made from two day old embryos, OPCs have been engineered toward being a single cell type, an oiigodendrocyte, a type of nerve support cell.
These are the same human cells Christopher Reeve raved about in a November 2004 television ad he taped just days before he died. Reeve was endorsing Prop 71, a California ballot measure to sell $6 billion in bonds that created the California Institute for Regenerative Medicine (CIRM), the state stem cell agency. "Stem cells have already cured paralysis in animals," Reeve said, showing some once gimpy rats with newfound ambulatory friskiness..
The animals in the Reeve commercial came from UC Irvine scientist Hans Keirstead. He had treated them with human OPCs supplied by the California company Geron.
The backstory here deserves mention, since this all started with SCI community advocacy. Keirstead got his initial funding to study OPCs by way of the Roman Reed Spinal Cord Injury Research Act, a California program that funded an average of $1.5 million a year in SCI research for ten years, starting in 2001. Reed, from the Bay Area, was injured playing football in 1994. He and his dad, Don, led a vigorous grassroots effort to line up legislative backing for the SCI funding.
The who’s who of SCI scientists in California got Reed grants, including Reggie Edgerton, Susan Harkema, Mark Tuszynski, Michael Softoniew, Kim Anderson, Candace Floyd, and Os Steward. There were 129 grants total, which produced data that was leveraged to get $64 million in new money from NIH or outside sources.
In 2002, Keirstead received $92,484 for “The remyelinating potential of human embryonic stem cell-derived oligodendrocytes.” He soon had data, some of which was preemptively shared in the 2004 Reeve/CIRM promo.

Christopher Reeve discusses the potential benefits of stem cell research at a neuroscience conference at MIT on March 2, 2003.
History will note that Keirstead jumped the customary gun on revealing study results – he had yet to have his preclinical OPC data peer reviewed – sort of a cowboy move in academic research. Keirstead’s OPC study did come along a few months later (20 years ago this month) and reported enough recovery for Geron to jump straight ahead into a human OPC trial, the first ever clinical use of embryonic stem cells.
The stem cell field is still young in science terms, less than 30 years old. James Thomson and colleagues at the University of Wisconsin got it started, publishing the groundbreaking paper (Embryonic stem cell lines derived from human blastocysts) in 1998. They demonstrated that a pluripotent line of stem cells could be made from a two day old human embryo and that these new cells could differentiate into any cell type in the body.
Buried in the detail of Thomson’s paper, he acknowledged financial support from the U of W, and from the then-unknown cancer biotech company, Geron. The company was in on the stem cell gambit very early on.
The Thomson paper set off a huge controversy and was quickly politicized: The Bush administration banned NIH funding for embryonic stem cells in 2001. But not these OPC cells – these were derived from one of 71 existing cell lines Bush allowed.
And so, to circle back to CIRM, it was the Bush restrictions that fueled widespread public support in California to fund stem cell research without federal interference. It was Keirstead’s paper that helped sell CIRM to voters. And then, CIRM comes back around to provided $14.4 million to Geron and its heirs to keep the OPC cells relevant.
The First OPC Trial
Geron faced strong regulatory headwinds to get the trial going. Two clinical holds were placed on the application pending fears of tumor formation. Geron says it spent $45 million on its 22,000-page application, reportedly the largest the FDA had ever received. They finally got the green light in the summer of 2010; in October, the first participant, a young paraplegic man injured just a week, got the first OPCs, in Atlanta.
Geron dosed four more individuals with acute thoracic SCI before suddenly abandoning the effort in 2011 to focus on cancer therapies. This was a time of great hope, and of course great hype and unrealistic expectation, for stem cells; the Geron fail was shocking news.

Not so fast. Two former Geron execs worked out a deal to acquire the OPC cell line and early trial data. They started Asterias, which in a clinical trial called SCIStar implanted OPCs in 25 more people, including sub-acute (three to six weeks post injury) and cervical injuries.
Participants were ASIA A (motor and sensory complete) or AISA B (motor complete, sensory incomplete). The early patients got two million cells; the dose escalated to 20 million in the later cohorts.

In 2022 Asterias merged with a San Diego company that changed its name from BioTime to Lineage Cell Therapeutics. That’s who is sponsoring the new trial. Lineage is not looking at how well the cells work in terms of recovery of function. They’re looking at safety, and whether using a new surgical cell delivery device improves safety.
End note
My work at U2FP this year is funded by Bob Yant and Ann Kenowsky, and I thank them very much for their support. Bob and his wife, Ann, have been friends for 40 years; Bob lives with a C5/6 injury and remains a tireless battler for therapy development. He was a Board Director for the American Paralysis Association, which became the Reeve Foundation. Bob was also on the External Advisory Board to oversee Roman Reed State Funds, which kickstarted the original OPC studies. Recently Bob founded two biotech companies with an eye on SCI therapies.


















