Apr 24, 2024
Headline Patrol: In Mayo Stem Trial 7 of 10 Got Better, Especially One
Sam Maddox
Mayo Clinic recently published results of a 10-patient study using stem cells for chronic spinal cord injury. The cells are drawn from the stomach fat of each patient, processed, and injected into the spinal fluid. The so-called Celltop trial reported that 7 of 10 got a measurable return of sensory or motor function.
Let’s break this down a little. First, it’s good news when a trial for chronic SCI meets its endpoint, in this case the goal was to avoid adverse events (AE) acutely and over time. There were AEs, mostly musculoskeletal pain and headaches, but none were deemed serious.
Second, any bit of neural recovery is of course very newsworthy. Except for one patient, the improvements were modest – increased strength in muscle groups and increased sensation to light touch and pinpricks. This is cool because the stem cells seem to have had an effect, and it means the study will continue to test 40 more patients (Celltop II, this time the primary outcome is more challenging: they’re hoping for recovery, with secondary measures for bladder and bowel function).
It’s the one participant who got a lot back that continues to make the Mayo study a big headline generator. Let’s catch up with Chris Barr, whose story we covered in late 2019.

From left: Mohamed Bydon, Chris Barr and his wife, Debbie.
Barr broke his neck in 2017 surfing in Northern California; he was diagnosed as AIS A, meaning complete motor and sensory paralysis below his neck. After a decompression surgery there was recovery – he had converted to AIS C, meaning he could move his extremities to some degree. Barr was thinking that might be it for getting anything else back. Without any expectations he enrolled in Celltop, a small experimental stem cell trial at Mayo.
Nine months post injury, Barr, then 53, came to Rochester, was injected with 100 million of his own processed fat cells, and said he could feel an effect right away. He got a lot more recovery, and could eventually walk unassisted.
When neurosurgeon and principal investigator Mohamad Bydon published a case study paper on the results, Barr got the full blast “paralyzed man walks” media treatment. He was interviewed by Christopher Reeve’s son Will on ABC’s Good Morning America. See: Man in wheelchair from paralysis walks again thanks to a new medical innovation.
Bydon was as surprised as anyone. He called Barr a “super-responder.” He and his team don’t know why cells worked so well in Barr and in others not at all. Dosing? Timing?
(For more on Bydon, check out the U2FP Curecast interview from early 2020.)
The Latest ‘Man Walks’ Coverage
After the full Celltop study results were published earlier this month, Barr was once more all over the news, and again on ABC’s morning show with Reeve. He still stands, and walks even faster than five years ago. Here are a couple of recent news samples, in case you missed the coverage:

Chris Barr seen on ABC's Good Morning America walking after stem cell treatment for his spinal cord injury (ABC News/GMA)

Chris Barr seen on ABC's Good Morning America walking after stem cell treatment for his spinal cord injury (ABC News/GMA)
Here at U2FP we’ve come to know Chris Barr very well as a supporter and colleague. He is an articulate and committed advocate for better, smarter research and has contributed to several academic studies, one on why there are so many clinical trials, yet still no therapies (which I reported on, here). He’s currently collaborating on another U2FP-facilitated study, in progress, on where the money from SCI funders is actually going.
Barr is of course thrilled with his super-response, though it’s not like the dragon of his paralysis has been slain; he still deals with all sorts of SCI-related secondary conditions and has not stopped seeking better therapies. I asked him to put this small stem cell trial into perspective. Said Barr:
What’s interesting to me is that the benefit of the stem cells is in the ballpark for the benefits of spinal cord stimulation – one third get material recovery, one third some, one third none. Both interventions mostly benefit incompletes. No one really knows why either of those interventions work, e.g. definitive mechanisms. And no one knows why they don’t work on everyone.
As always, we still need more research, but now that there are bonafide published results my hope would be for an aggressive increase in testing and bold research incorporating combinational interventions.
First, the Money
Before drilling down into the science, let’s not forget where the financial support came from to start Celltop.
A major part of the funding came from the state of Minnesota, which therefore links it back to the SCI community. Prodded by SCI activists (initially led by Matthew Rodreick, now Executive Director of U2FP) the state passed legislation in 2015 allocating $1 million for SCI and brain injury research. The next year, they added $6 million more to their biennium budget, which has continued year over year, ever since. Mayo received one of the first grants from the program.
Minnesota’s advocacy success has become a template for U2FP efforts in other states. Almost $35 million of grassroots money has been funded by states so far. To learn more, see U2FP's Cure Advocacy Network page, here.
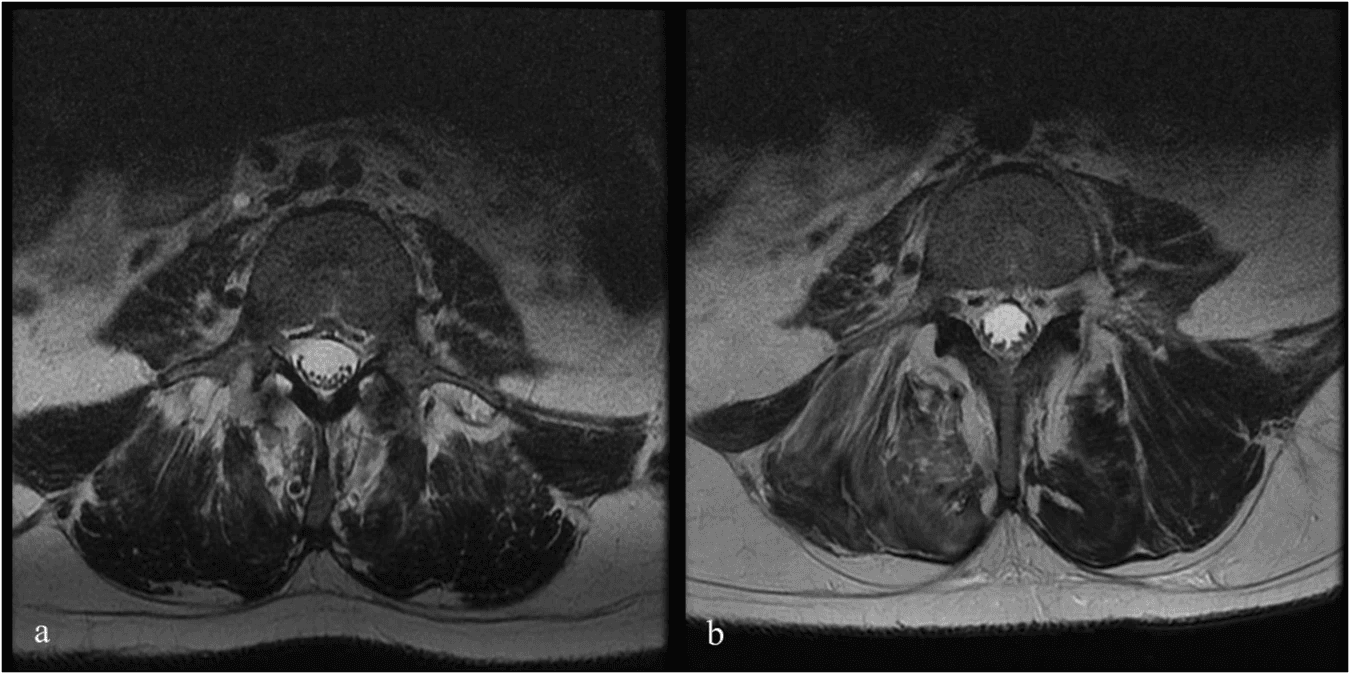
Fig. 1: Pre- and Post-Infusion MRI of Patient 6. Bydon, M., Qu, W., Moinuddin, F.M. et al. Intrathecal delivery of adipose-derived mesenchymal stem cells in traumatic spinal cord injury: Phase I trial. Nat Commun 15, 2201 (2024). https://doi.org/10.1038/s41467-024-46259-y
Celltop: The Science
Here are the specific patient profiles and enrollment details on the Celltop study, from the published paper:
All patients were enrolled within 12 months of injury (range 2–12 months). Six of the patients had a cervical-level injury, and four had a thoracic-level injury. At the time of injury, eight patients were classified as AIS grade A and two patients as AIS grade B
Five patients were classified as AIS A at the time of injection. Of those, two remained AIS A at the last follow-up, while three changed from AIS A to B (two patients) and to AIS C (one patient) at week 96. Four of the five patients that were classified as AIS B or C at injection demonstrated improvement in AIS grade.
Why fat cells? They are easy to get, easy to process, and seem to have a good track record for safety and effect:
Adipose tissue represents the most prominent reservoir of mesenchymal stem cells, named adipose-derived mesenchymal stem cells (AD-MSCs). Adipose-derived MSCs are considered attractive options due to their availability, ease of access, and multipotency. The use of AD-MSC has been thoroughly investigated in traumatic and degenerative diseases. Preclinical trials in SCI animal models have found evidence that AD-MSCs can regulate the inflammatory response and promote a regenerative environment, correlating with promising clinical outcomes.
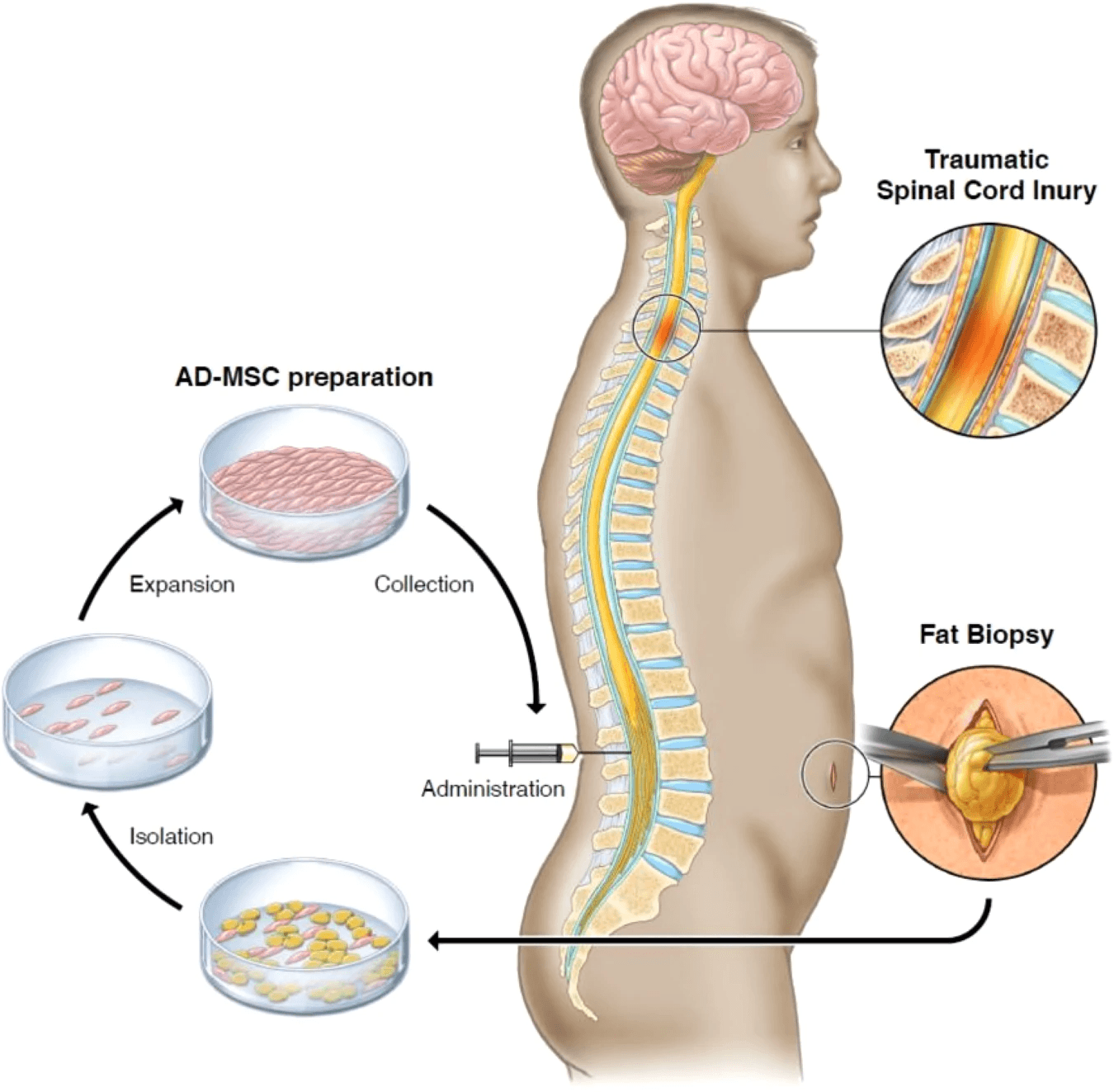
Fig. 6: Fat harvest via biopsy, AD-MSCs isolation, expansion, and collection, and treatment administration. Bydon, M., Qu, W., Moinuddin, F.M. et al. Intrathecal delivery of adipose-derived mesenchymal stem cells in traumatic spinal cord injury: Phase I trial. Nat Commun 15, 2201 (2024). https://doi.org/10.1038/s41467-024-46259-y
What do these stem cells do? They don’t seem to stick around very long in the spinal cord area so it is not likely they are replacing damaged nerve cells. Perhaps the transplanted cells cultivate a more hospitable habitat for nerve, blood and immune recovery. From the paper:
Currently, there is a substantial body of evidence generated from preclinical studies indicating MSCs may potentially modulate various pathways involved in endogenous neurogenesis, angiogenesis, immunological regulation, and neuronal plasticity. Compared with other adult stem cells, AD-MSCs are particularly advantageous, given their angiogenic and neuro-regenerative capacity, in addition to their pluripotency, ease of harvest, and availability.
Preclinical and clinical studies suggest that MSC preservation in vivo is limited, and their favorable effects may be due to their ability to regulate tissue homeostasis and inflammation through the synthesis of various paracrine factors [localized effect]. In this study, we observed an increase in the level of VEGF [vascular endothelial growth factor]. This supports the proposed paracrine mechanism of action of MSCs in SCI; however, further investigation is needed to explore this association and potential mediating process.
More work to do. Here’s Bydon, upbeat but urging patience, highlighting that we’re still at the beginning of the stem cell therapy journey. From a Mayo release:
For years, treatment of spinal cord injury has been limited to supportive care, more specifically stabilization surgery and physical therapy. Many historical textbooks state that this condition does not improve. In recent years, we have seen findings from the medical and scientific community that challenge prior assumptions. This research is a step forward toward the ultimate goal of improving treatments for patients.
Spinal cord injury is a complex condition. Future research may show whether stem cells in combination with other therapies could be part of a new paradigm of treatment to improve outcomes for patients.
File this study result under encouraging. Overall, it’s not a knockout but it’s a win. It was safe. Now let’s see how the next phase goes, with more participants. Meanwhile we’ll keep an eye on the cell therapy development front.

















